Services
Trademarks
Protect your brand today
Register your Trademark
Post Filing Services
Trademark Protection
Patents
File a patent today
Start a Patent Application
Copyrights
Safeguard your creative ideas
Start a Copyright Application
Business
Incorporate your business today
Start a Business
Legal Forms for Business
Resources
Guest
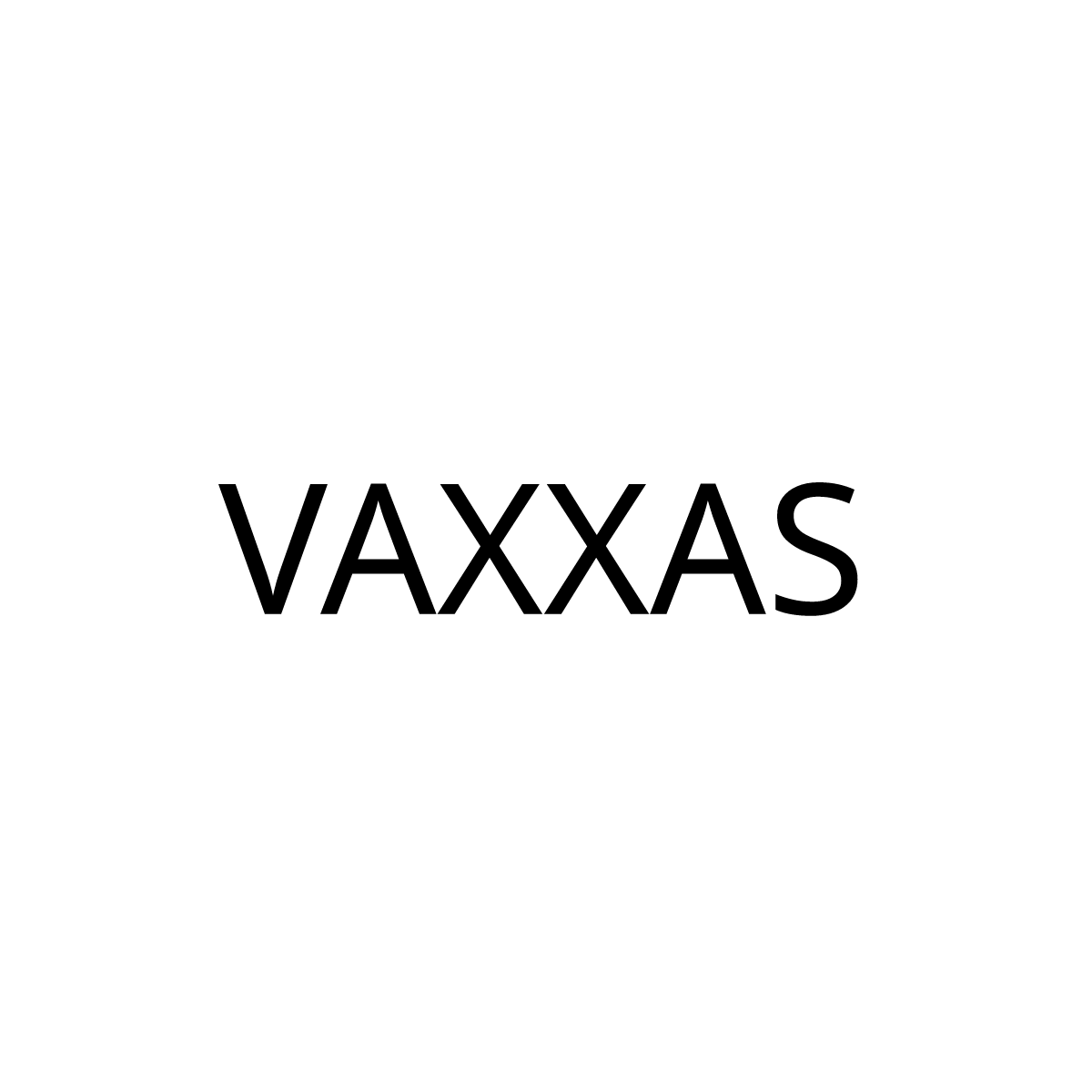
REGISTERED
on 10 Nov 2023
Last Applicant/ Owned by
VAXXAS PTY LTD
c/- One Ventures Management, Suite 13.02,179 Elizabeth StSydney NSW 2000
AU
Serial Number
2034816 filed on 20th Apr 2020
Registration Number
TMA1208145 registered on 10th Nov 2023
Registration expiry Date
20th Apr 2030Correspondent Address
550 BURRARD STREETSUITE 2300VANCOUVER
BRITISH COLUMBIA
CA
V6C2B5
VAXXAS
Trademark usage description
pharmaceutical, medicinal and therapeutic preparations for human and veterinary use for the treatment of infectious diseases, namely, anthrax, bat lys Read MoreClassification Information
Class [005]
Pharmaceutical, medicinal and therapeutic preparations for human and veterinary use for the treatment of infectious diseases, namely, anthrax, bat lyssavirus infection, avian influenza, botulism, brucellosis, campylobacteriosis, chickenpox, chikungunya virus, chlamydial infection, cholera, COVID-19, Creutzfeldt- Jakob disease, cryptosporidiosis, dengue virus infection, diphtheria, donovanosis, encephalitis, flavivirus, gonococcal, streptococcal disease, haemolytic uraemic syndrome, haemophilus influenzae type b, hepatitis, hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E, human immunodeficiency virus, human papillomavirus, influenza, Japanese encephalitis, legionellosis, leprosy, leptospirosis, listeriosis, lyssavirus infection, malaria, measles, meningococcal disease, Middle East respiratory syndrome, monkeypox, mumps, paratyphoid fever, pertussis, plague, pneumococcal disease, polio, psittacosis, ornithosis, Q fever, rabies, respiratory syncytial virus, rotavirus, rubella, salmonellosis, severe acute respiratory syndrome, shiga toxin producing Escherichia coli, shigellosis, shingles, smallpox, syphilis, tetanus, tuberculosis, tularaemia, typhoid fever, varicella zoster infection, viral haemorrhagic fever, West Nile virus infection, Yellow fever, allergies and oncology, human and animal vaccines, human and animal vaccine preparations, diagnostic reagents for medical and veterinary diagnostic use, biological tissue cultures for medical purposes, blood and plasma for human medical diagnostics and analysis, transdermal patches, bandages, poultices and medical dressings impregnated with vaccines, patches, bandages, poultices and medical dressings impregnated with vaccines, transdermal vaccine delivery system being patches for administering vaccines, pharmaceutical preparations for treating infectious diseases, namely, anthrax, bat lyssavirus infection, avian influenza, botulism, brucellosis, campylobacteriosis, chickenpox, chikungunya virus, chlamydial infection, cholera, COVID-19, Creutzfeldt-Jakob disease, cryptosporidiosis, dengue virus infection, diphtheria, donovanosis, encephalitis, flavivirus, gonococcal, streptococcal disease, haemolytic uraemic syndrome, haemophilus influenzae type b, hepatitis, hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E, human immunodeficiency virus, human papillomavirus, influenza, Japanese encephalitis, legionellosis, leprosy, leptospirosis, listeriosis, lyssavirus infection, malaria, measles, meningococcal disease, Middle East respiratory syndrome, monkeypox, mumps, paratyphoid fever, pertussis, plague, pneumococcal disease, polio, psittacosis, ornithosis, Q fever, rabies, respiratory syncytial virus, rotavirus, rubella, salmonellosis, severe acute respiratory syndrome, shiga toxin producing Escherichia coli, shigellosis, shingles, smallpox, syphilis, tetanus, tuberculosis, tularaemia, typhoid fever, varicella zoster infection, viral haemorrhagic fever, West Nile virus infection, Yellow fever, allergies and oncology contained in transdermal patches, skin patches for the transdermal delivery of pharmaceuticals for use in the treatment of infectious diseases, namely, anthrax, bat lyssavirus infection, avian influenza, botulism, brucellosis, campylobacteriosis, chickenpox, chikungunya virus, chlamydial infection, cholera, COVID-19, Creutzfeldt-Jakob disease, cryptosporidiosis, dengue virus infection, diphtheria, donovanosis, encephalitis, flavivirus, gonococcal, streptococcal disease, haemolytic uraemic syndrome, haemophilus influenzae type b, hepatitis, hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E, human immunodeficiency virus, human papillomavirus, influenza, Japanese encephalitis, legionellosis, leprosy, leptospirosis, listeriosis, lyssavirus infection, malaria, measles, meningococcal disease, Middle East respiratory syndrome, monkeypox, mumps, paratyphoid fever, pertussis, plague, pneumococcal disease, polio, psittacosis, ornithosis, Q fever, rabies, respiratory syncytial virus, rotavirus, rubella, salmonellosis, severe acute respiratory syndrome, shiga toxin producing Escherichia coli, shigellosis, shingles, smallpox, syphilis, tetanus, tuberculosis, tularaemia, typhoid fever, varicella zoster infection, viral haemorrhagic fever, West Nile virus infection, Yellow fever, allergies and oncology, transdermal patches for administering pharmaceuticals for use in the treatment of infectious diseases, namely, anthrax, bat lyssavirus infection, avian influenza, botulism, brucellosis, campylobacteriosis, chickenpox, chikungunya virus, chlamydial infection, cholera, COVID-19, Creutzfeldt-Jakob disease, cryptosporidiosis, dengue virus infection, diphtheria, donovanosis, encephalitis, flavivirus, gonococcal, streptococcal disease, haemolytic uraemic syndrome, haemophilus influenzae type b, hepatitis, hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E, human immunodeficiency virus, human papillomavirus, influenza, Japanese encephalitis, legionellosis, leprosy, leptospirosis, listeriosis, lyssavirus infection, malaria, measles, meningococcal disease, Middle East respiratory syndrome, monkeypox, mumps, paratyphoid fever, pertussis, plague, pneumococcal disease, polio, psittacosis, ornithosis, Q fever, rabies, respiratory syncytial virus, rotavirus, rubella, salmonellosis, severe acute respiratory syndrome, shiga toxin producing Escherichia coli, shigellosis, shingles, smallpox, syphilis, tetanus, tuberculosis, tularaemia, typhoid fever, varicella zoster infection, viral haemorrhagic fever, West Nile virus infection, Yellow fever, allergies and oncology and vaccines, intradermal patches for use in the medical treatment of infectious diseases, namely, anthrax, bat lyssavirus infection, avian influenza, botulism, brucellosis, campylobacteriosis, chickenpox, chikungunya virus, chlamydial infection, cholera, COVID-19, Creutzfeldt-Jakob disease, cryptosporidiosis, dengue virus infection, diphtheria, donovanosis, encephalitis, flavivirus, gonococcal, streptococcal disease, haemolytic uraemic syndrome, haemophilus influenzae type b, hepatitis, hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E, human immunodeficiency virus, human papillomavirus, influenza, Japanese encephalitis, legionellosis, leprosy, leptospirosis, listeriosis, lyssavirus infection, malaria, measles, meningococcal disease, Middle East respiratory syndrome, monkeypox, mumps, paratyphoid fever, pertussis, plague, pneumococcal disease, polio, psittacosis, ornithosis, Q fever, rabies, respiratory syncytial virus, rotavirus, rubella, salmonellosis, severe acute respiratory syndrome, shiga toxin producing Escherichia coli, shigellosis, shingles, smallpox, syphilis, tetanus, tuberculosis, tularaemia, typhoid fever, varicella zoster infection, viral haemorrhagic fever, West Nile virus infection, Yellow fever, allergies and oncology, intradermal vaccine delivery system being patches for administering human and animal vaccines, pharmaceutical preparations contained in intradermal patches for use in the medical treatment of infectious diseases, namely, anthrax, bat lyssavirus infection, avian influenza, botulism, brucellosis, campylobacteriosis, chickenpox, chikungunya virus, chlamydial infection, cholera, COVID-19, Creutzfeldt-Jakob disease, cryptosporidiosis, dengue virus infection, diphtheria, donovanosis, encephalitis, flavivirus, gonococcal, streptococcal disease, haemolytic uraemic syndrome, haemophilus influenzae type b, hepatitis, hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E, human immunodeficiency virus, human papillomavirus, influenza, Japanese encephalitis, legionellosis, leprosy, leptospirosis, listeriosis, lyssavirus infection, malaria, measles, meningococcal disease, Middle East respiratory syndrome, monkeypox, mumps, paratyphoid fever, pertussis, plague, pneumococcal disease, polio, psittacosis, ornithosis, Q fever, rabies, respiratory syncytial virus, rotavirus, rubella, salmonellosis, severe acute respiratory syndrome, shiga toxin producing Escherichia coli, shigellosis, shingles, smallpox, syphilis, tetanus, tuberculosis, tularaemia, typhoid fever, varicella zoster infection, viral haemorrhagic fever, West Nile virus infection, Yellow fever, allergies and oncology, skin patches for the intradermal delivery of pharmaceuticals for use in the treatment of infectious diseases, namely, anthrax, bat lyssavirus infection, avian influenza, botulism, brucellosis, campylobacteriosis, chickenpox, chikungunya virus, chlamydial infection, cholera, COVID-19, Creutzfeldt-Jakob disease, cryptosporidiosis, dengue virus infection, diphtheria, donovanosis, encephalitis, flavivirus, gonococcal, streptococcal disease, haemolytic uraemic syndrome, haemophilus influenzae type b, hepatitis, hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E, human immunodeficiency virus, human papillomavirus, influenza, Japanese encephalitis, legionellosis, leprosy, leptospirosis, listeriosis, lyssavirus infection, malaria, measles, meningococcal disease, Middle East respiratory syndrome, monkeypox, mumps, paratyphoid fever, pertussis, plague, pneumococcal disease, polio, psittacosis, ornithosis, Q fever, rabies, respiratory syncytial virus, rotavirus, rubella, salmonellosis, severe acute respiratory syndrome, shiga toxin producing Escherichia coli, shigellosis, shingles, smallpox, syphilis, tetanus, tuberculosis, tularaemia, typhoid fever, varicella zoster infection, viral haemorrhagic fever, West Nile virus infection, Yellow fever, allergies and oncology, intradermal patches for medical treatment for administering pharmaceuticals for use in the medical treatment of infectious diseases, namely, anthrax, bat lyssavirus infection, avian influenza, botulism, brucellosis, campylobacteriosis, chickenpox, chikungunya virus, chlamydial infection, cholera, COVID-19, Creutzfeldt-Jakob disease, cryptosporidiosis, dengue virus infection, diphtheria, donovanosis, encephalitis, flavivirus, gonococcal, streptococcal disease, haemolytic uraemic syndrome, haemophilus influenzae type b, hepatitis, hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E, human immunodeficiency virus, human papillomavirus, influenza, Japanese encephalitis, legionellosis, leprosy, leptospirosis, listeriosis, lyssavirus infection, malaria, measles, meningococcal disease, Middle East respiratory syndrome, monkeypox, mumps, paratyphoid fever, pertussis, plague, pneumococcal disease, polio, psittacosis, ornithosis, Q fever, rabies, respiratory syncytial virus, rotavirus, rubella, salmonellosis, severe acute respiratory syndrome, shiga toxin producing Escherichia coli, shigellosis, shingles, smallpox, syphilis, tetanus, tuberculosis, tularaemia, typhoid fever, varicella zoster infection, viral haemorrhagic fever, West Nile virus infection, Yellow fever, allergies and oncology and vaccines, microprojection array patches for medical purposes impregnated with vaccines, structural parts and fittings for the aforesaid goods.
Classification kind code
11
Class [010]
Surgical and medical apparatus and instruments namely, vaccine and drug delivery microneedle array patches sold without medication, drug delivery devices and apparatus, namely, hermetically sealed applicators containing patches for applying vaccine sold without medication, drug delivery devices sold empty, namely, uncoated patches sold without medication, transdermal drug delivery devices and apparatus namely, transdermal drug delivery patches sold without medication, intradermal drug delivery devices and apparatus, namely, intradermal drug delivery patches sold without medication, micro-projection drug delivery devices and apparatus, namely, drug delivery patches sold without medication, structural parts, fittings for the aforesaid goods.
Classification kind code
11
Class [040]
Custom manufacturing services in the field of drug delivery patches, custom manufacture of medical devices, pharmaceuticals, diagnostic tests and testing kits, custom manufacture of micro-projection array patches, custom manufacture of drug delivery devices and apparatus, custom manufacture of transdermal drug delivery devices and apparatus, custom manufacture of intradermal drug delivery devices and apparatus, custom manufacture of micro-projection drug delivery devices and apparatus, advisory, consultancy and information services in relation to all of the aforesaid.
Classification kind code
11
Class [042]
Research, design and development services in the field of medical apparatus and instruments, medical research, design and development services in the field of drug delivery devices, research and development in the pharmaceutical and biotechnology fields, research and development of vaccines and medicines, pharmaceutical drug development services, vaccine screening, namely safety testing of vaccines, scientific and technological research services related to immunopharmaceuticals and vaccines, biological research, clinical research in the field of immunology, vaccines, and medical research, conducting clinical trials for others, providing medical and scientific research information in the field of pharmaceuticals and clinical trials, chemical and biochemical analysis services, medical research, pharmaceutical research services, laboratory testing services in the field of vaccines, pharmaceuticals, laboratory research in the fields of biotechnology, microbiology, molecular biology, nanotechnology analytical chemistry, biochemistry, medicinal chemistry and drug development, technology services, namely, design and development of computer hardware, scientific research, clinical research, development, advisory and consultancy services in relation to antibody production, research and development services in the field of antibodies, antibody generation services being research and development services, diagnostic and research services and screening in the field of infectious disease, allergies and oncology for medical research purposes, diagnostic testing services in the field of medical apparatus and instruments, medical research services, advisory services relating to diagnostic testing of pharmaceutical drugs for quality control and safety purposes, research and development of pharmaceutical drugs, advisory, consultancy and information services in relation to all of the aforesaid.
Classification kind code
11
Class [044]
Medical services in the nature of administering human vaccines and providing medical information regarding human vaccines, healthcare services for providing and administering human vaccines, advisory and consultancy services in relation to clinical medical and veterinary services, medical testing for diagnostic or treatment purposes, advisory, consultancy and information services in relation to all of the aforesaid.
Classification kind code
11
Class [045]
Legal services relating to the exploitation and licensing of intellectual property and technology, licensing of medical technology, legal services relating to the licensing of research and development of products, advisory, consultancy and information services in relation to all of the aforesaid.
Classification kind code
11
Mark Details
Serial Number
2034816
Mark Type
Trademark
Legal History
| Action Taken | Status |
|---|---|
| Submitted for opposition 22 on 15th Oct 2021 | Search Recorded |
| Submitted for opposition 20 on 15th Oct 2021 | Examiner's First Report |
| Submitted for opposition 223 on 15th Oct 2021 | Total Provisional Refusal |
| Submitted for opposition 256 on 19th Apr 2021 | Notification of Possible Opposition Sent |
| Submitted for opposition 48 on 15th Sep 2020 | Agent Changed |
| Submitted for opposition 257 on 18th Jun 2020 | Designation Notification - Madrid Protocol |
| Submitted for opposition 1 on 17th Jun 2020 | Created |
| Submitted for opposition 31 on 17th Jun 2020 | Formalized |
| Submitted for opposition 30 on 20th Apr 2020 | Filed |
| Submitted for opposition 228 on 20th Apr 2020 | International Registration |