Services
Trademarks
Protect your brand today
Register your Trademark
Post Filing Services
Trademark Protection
Patents
File a patent today
Start a Patent Application
Copyrights
Safeguard your creative ideas
Start a Copyright Application
Business
Incorporate your business today
Start a Business
Legal Forms for Business
Resources
Guest
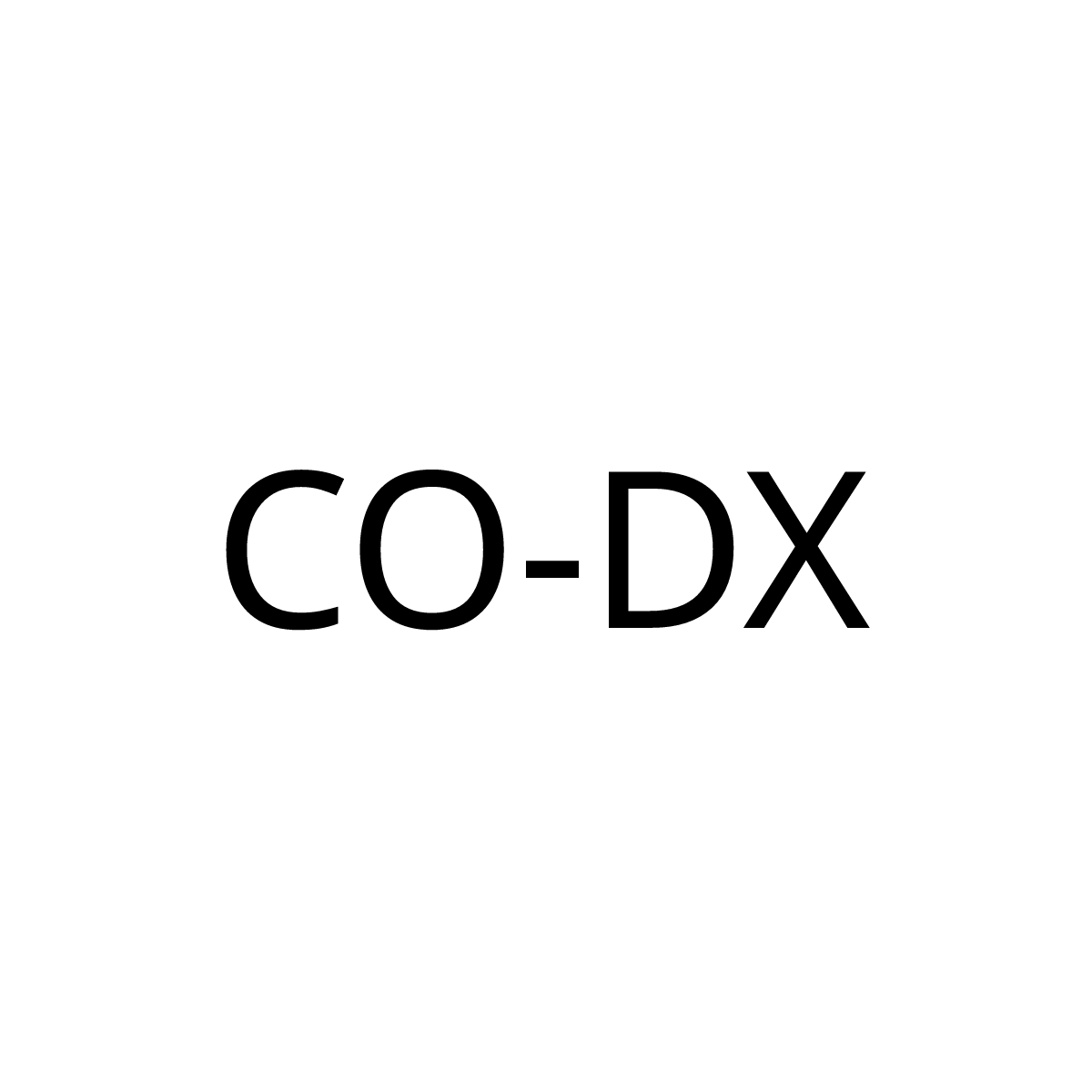
FORMALIZED
on 11 Sep 2024
Last Applicant/ Owned by
CO-DIAGNOSTICS INC.
2401 South Foothill Drive, Suite DSalt Lake City UT 84109
US
Serial Number
2349129 filed on 07th Jun 2024
Correspondent Address
630, boul. René-Lévesque Ouest20e étageMontréal
QUÉBEC
CA
H3B1S6
CO-DX
Trademark usage description
reagent kits comprising dna and rna primers, dna and rna probes, polymerase and buffers for use in pcr analysis for industrial or scientific purposes; Read MoreClassification Information
Class [001]
Reagent kits comprising DNA and RNA primers, DNA and RNA probes, polymerase and buffers for use in PCR analysis for industrial or scientific purposes; reagent kits comprising RNA primers and probes, polymerase and buffers for use in PCR analysis for industrial or scientific purposes; reagent kits comprising DNA and RNA primers, DNA and RNA probes, polymerase and buffers for use in detection of DNA and RNA in a sample for industrial or scientific purposes; reagent kits comprising RNA primers and probes, polymerase and buffers for use in detection of RNA in a sample for industrial or scientific purposes.
Classification kind code
12
Class [005]
Reagent kits comprising DNA and RNA primers, DNA and RNA probes, polymerase and buffers for use in PCR analysis for medical or veterinary purposes; reagent kits comprising RNA primers and probes, polymerase and buffers for use in PCR analysis for medical or veterinary purposes; reagent kits comprising RNA primers and probes, polymerase and buffers for use in detection of RNA in a sample for medical or veterinary purposes.
Classification kind code
12
Class [009]
Downloadable software, namely, mobile application software for receiving medical testing data and providing medical testing results; downloadable software, namely, mobile application software for receiving DNA and RNA testing data and providing DNA and RNA testing results; downloadable software, namely, mobile application software for receiving RNA testing data and providing RNA testing results; downloadable software, namely, mobile application software for receiving DNA testing data and providing DNA testing results.
Classification kind code
12
Class [010]
Medical apparatus, namely, a device for analysis of DNA and RNA in a sample; medical apparatus, namely, a device for analysis of RNA in a sample; medical apparatus, namely, a device for detection of DNA and RNA in a sample; medical apparatus, namely, a device for detection of RNA in a sample.
Classification kind code
12
Mark Details
Serial Number
2349129
Mark Type
Trademark
Legal History
| Action Taken | Status |
|---|---|
| Submitted for opposition 48 on 16th Oct 2024 | Agent Changed |
| Submitted for opposition 257 on 12th Sep 2024 | Designation Notification - Madrid Protocol |
| Submitted for opposition 1 on 11th Sep 2024 | Created |
| Submitted for opposition 31 on 11th Sep 2024 | Formalized |
| Submitted for opposition 228 on 07th Jun 2024 | International Registration |
| Submitted for opposition 30 on 07th Jun 2024 | Filed |