Services
Trademarks
Protect your brand today
Register your Trademark
Post Filing Services
Trademark Protection
Patents
File a patent today
Start a Patent Application
Copyrights
Safeguard your creative ideas
Start a Copyright Application
Business
Incorporate your business today
Start a Business
Legal Forms for Business
Resources
Guest
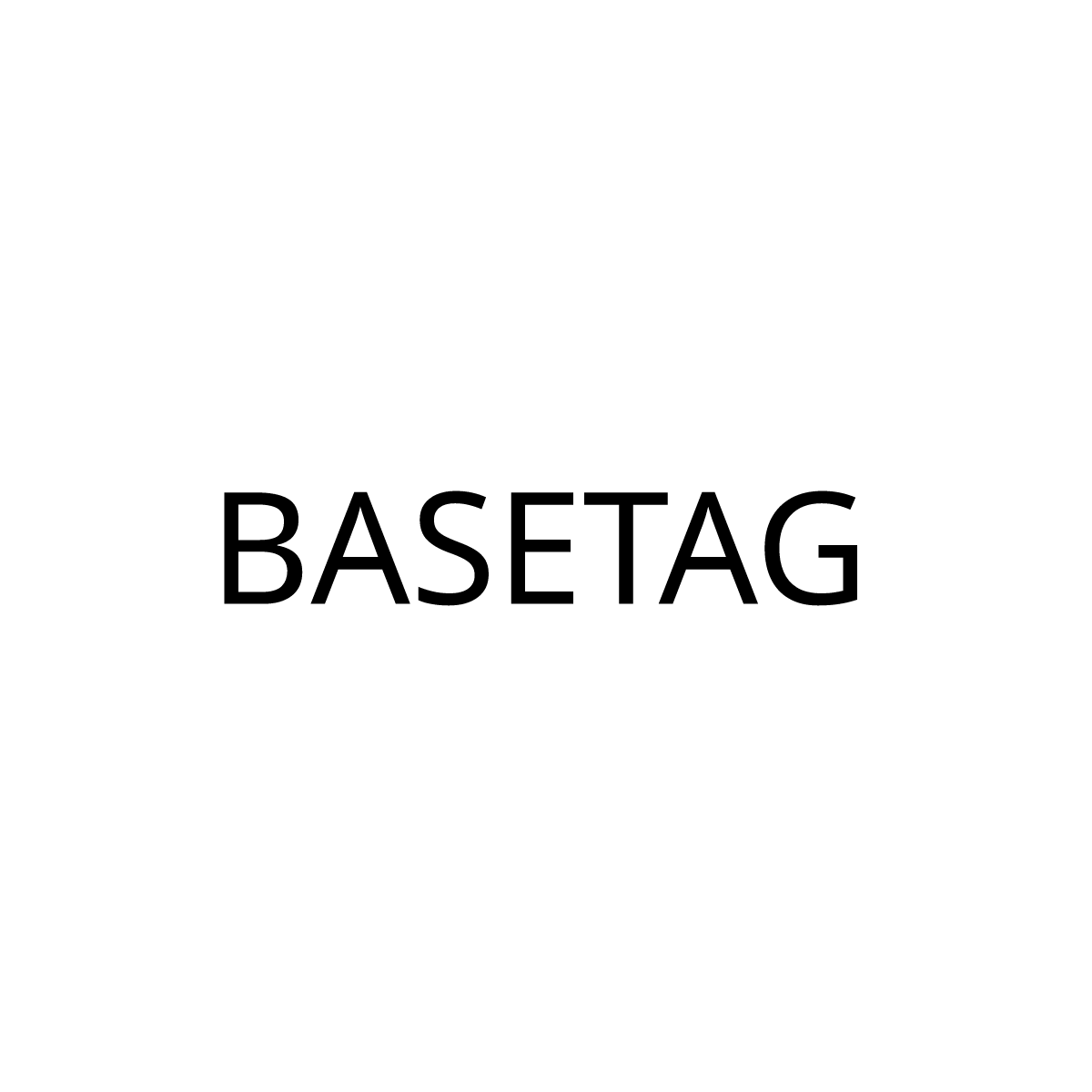
REGISTERED
on 27 Sep 2023
Last Applicant/ Owned by
ROCHE DIAGNOSTICS GMBH
Sandhofer Strasse 11668305 Mannheim
DE
Serial Number
2059223 filed on 08th Sep 2020
Registration Number
TMA1199887 registered on 27th Sep 2023
Registration expiry Date
08th Sep 2030Correspondent Address
SUITE 16001 FIRST CANADIAN PLACE100 KING STREET WESTTORONTO
ONTARIO
CA
M5X1G5
BASETAG
Trademark usage description
chemical, biological and biochemical preparations namely laboratory immunoassays, laboratory assays, monoclonal antibodies, cell culture media, cell g Read MoreClassification Information
Class [001]
Chemical, biological and biochemical preparations namely laboratory immunoassays, laboratory assays, monoclonal antibodies, cell culture media, cell growth media, stem cells all for scientific and research purposes; in vitro diagnostic reagents for use in industry and science; chemical additives, enzymes, reagents, organisms and proteins for use in nucleic acid amplification and detection for scientific and research use; in vitro diagnostics, namely chemical control solutions for calibrating and monitoring the accuracy and function of instruments and test results; chemical reagents for cell separation, labelling reagents; reagents for the processing, separation, isolation, enrichment, depletion, detection, screening, cultivation, analysis and counting of biological material; reagents with magnetic beads for scientific and research use; monoclonal antibody reagents for scientific purposes; diagnostic tests, consisting of reagents and chemical test kits for determining genetic information for scientific and research purposes; reagents and chemical test kits used for scientific and research purposes related to DNA sequencing and genetic analysis; biological assays and kits for use in fields of biological and diagnostic testing for industrial, scientific and research use; reagents and nucleotides for nucleic acid sequencing for use in science and research.
Classification kind code
11
Class [005]
Chemical, biological and biochemical preparations namely laboratory immunoassays, laboratory assays, monoclonal antibodies, cell culture media, cell growth media, stem cells all for medical purposes; in vitro diagnostic reagents for clinical and medical use and in vitro diagnostics, namely, reagents and control solutions for the analysis of biological samples for medical diagnostic purposes; in vitro diagnostic agents, namely, reagents and control solutions for analysis of biological samples for medical diagnostic purposes; chemical reagents for testing body fluids to determine chromosomal abnormalities; chemical reagents and test kits for medical, clinical and diagnostic purposes in the fields of DNA sequencing and genetics; diagnostic test kits consisting primarily of reagents for nucleic acid detection and analysis.
Classification kind code
11
Mark Details
Serial Number
2059223
Mark Type
Trademark
Legal History
| Action Taken | Status |
|---|---|
| Submitted for opposition 22 on 14th Feb 2022 | Search Recorded |
| Submitted for opposition 20 on 14th Feb 2022 | Examiner's First Report |
| Submitted for opposition 223 on 14th Feb 2022 | Total Provisional Refusal |
| Submitted for opposition 256 on 23rd Aug 2021 | Notification of Possible Opposition Sent |
| Submitted for opposition 48 on 19th Apr 2021 | Agent Changed |
| Submitted for opposition 257 on 22nd Oct 2020 | Designation Notification - Madrid Protocol |
| Submitted for opposition 1 on 21st Oct 2020 | Created |
| Submitted for opposition 31 on 21st Oct 2020 | Formalized |
| Submitted for opposition 30 on 08th Sep 2020 | Filed |
| Submitted for opposition 228 on 08th Sep 2020 | International Registration |