Services
Trademarks
Protect your brand today
Register your Trademark
Post Filing Services
Trademark Protection
Patents
File a patent today
Start a Patent Application
Copyrights
Safeguard your creative ideas
Start a Copyright Application
Business
Incorporate your business today
Start a Business
Legal Forms for Business
Resources
Guest
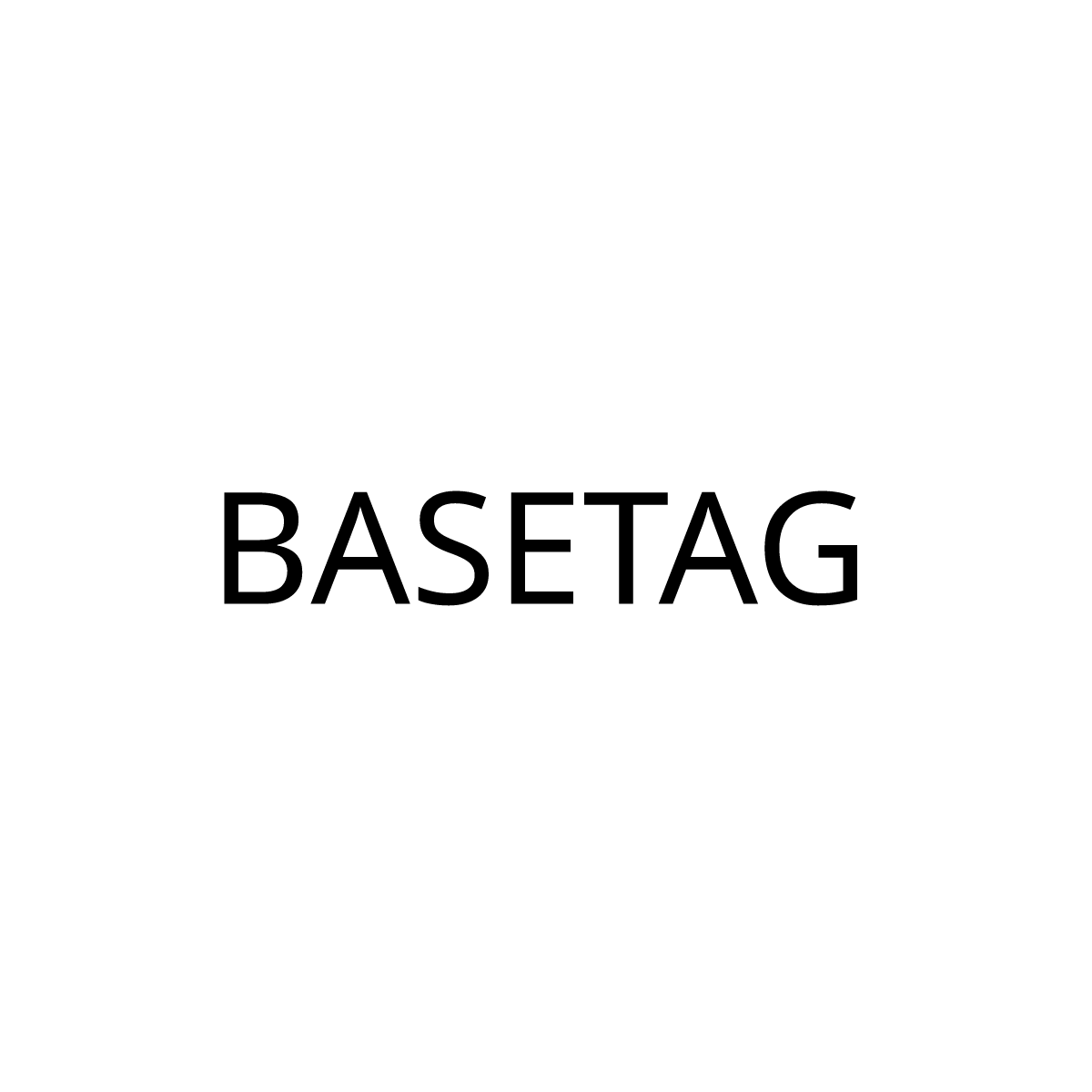
REGISTERED
on 04 Dec 2020
Last Applicant/ Owned by
ROCHE DIAGNOSTICS GMBH
Sandhofer Strasse 11668305 Mannheim
DE
Serial Number
1895446 filed on 24th Apr 2018
Registration Number
TMA1089185 registered on 04th Dec 2020
Registration expiry Date
04th Dec 2030Correspondent Address
SUITE 16001 FIRST CANADIAN PLACE100 KING STREET WESTTORONTO
ONTARIO
CA
M5X1G5
BASETAG
Trademark usage description
scientific laboratory instruments for measuring and data processing namely polymerase chain reaction amplifiers, prothrombin time blood testers, warfa Read MoreClassification Information
Class [009]
Scientific laboratory instruments for measuring and data processing namely polymerase chain reaction amplifiers, prothrombin time blood testers, warfarin testers, and blood coagulation testers for use in scientific research and clinical laboratory research use; in vitro diagnostics devices and instruments for identifying, analyzing, measuring and recording human DNA molecules for use in scientific research and clinical laboratory research use; In vitro diagnostics devices and instruments for identifying, analyzing, measuring, sequencing, and recording human biomolecules for use in scientific research and clinical laboratory research use; In vitro diagnostics devices and instruments for identifying, analyzing, measuring, sequencing, and recording human antigen and antibody molecules for research and clinical laboratory research use; laboratory devices for the detection of genomes, DNA, pathogens and toxins in a human biological sample for scientific research and for clinical laboratory research use; laboratory instruments for analyzing nucleic acids and for DNA sequencing for scientific research and for clinical laboratory research use; nucleic acid sequencers; computer software for image processing; image scanners; X-ray fluorescence analyzers; nanoparticle size analyzers; genotyping and gene expression microarrays, electronic microarray mapping files and microarray biochips for analyzing nucleic acids for scientific research, pharmaceutical research and medical research purposes; in vitro diagnostic software as a medical device to enable physicians, patients, hospitals, and medical clinics to monitor patient treatment progress, to operate artificial intelligence automation to rapidly connect the dots between patient symptoms, healthcare professional diagnosis, and evidence-based research in the field of biomarkers and disease indicators, clinical chemistry and immunoassays, molecular diagnostics, molecular sequencing for laboratory automation to support medical diagnosis in the field of scientific research, pharmaceutical research and medical research; computers and computer operating software for collecting, storing, analyzing and reporting human biological sample diagnosis information, and for sample tracking and managing projects, laboratory workflow and in vitro diagnostic information for use in the fields of scientific research, clinical diagnostic and research, and clinical laboratory diagnostic purposes; software for use in analyzing, managing and visualizing DNA sequencing data
Classification kind code
11
Class [010]
Medical instruments for general examination; blood collection bags for medical purposes; medical diagnostic instruments namely polymerase chain reaction amplifiers, prothrombin time blood testers, warfarin testers, and blood coagulation testers for use in medical and clinical laboratory use; medical devices and instruments for identifying, analyzing, measuring and recording human DNA molecules for medical and clinical laboratory use; medical devices and instruments for identifying, analyzing, measuring, sequencing, and recording human biomolecules for medical and clinical laboratory use; medical devices and instruments for identifying, analyzing, measuring, sequencing, and recording human antigen and antibody molecules; medical analytical apparatus namely polymerase chain reaction amplifiers, prothrombin time blood testers, warfarin testers, and blood coagulation testers for use in medical and clinical laboratory use; medical equipment for analyzing nucleic acids and for DNA sequencing for the purpose of rendering medical treatment and diagnosis
Classification kind code
11
Class [042]
Scientific laboratory research in the field of bacteriology, chemistry, DNA screening, genetic testing, hematology, biochemistry, biotechnology; consulting services in the field of bacteriology, chemistry, DNA screening, genetic testing, hematology, biochemistry, biotechnology; scientific research and consultancy services related thereto in the fields of genome sequence analysis and reporting; medical research services; providing DNA screening for scientific research purposes; providing laboratory diagnostic research services for others in the fields of cardiology, hematology, blood donor screening, coagulation, infectious diseases, gynecology, human tissue diagnostics, liquid biopsy, and genome and DNA sequencing; consulting services in the field of cloud computing providing software for database management in the fields of life science; technical support services, namely, computer network infrastructure management services for monitoring, administration and management of cloud computing operational software and database management software in the field of clinical laboratory research and diagnosis, scientific clinical and medical research, and pharmaceutical research; computer services, namely cloud computing web hosting provider services for storing, analyzing and sharing data in the fields of life science; providing online non-downloadable software for the custom design and ordering of assays and reagents; design and development of laboratory instruments and computer systems for use in science and clinical research; providing consultancy, information and advisory services in the field of analysis of biomolecules
Classification kind code
11
Class [044]
Medical services for analysis of genetics; genetic testing for medical purposes; nucleic acid based testing for medical purposes; medical services in the field of nucleic acid analysis; medical services in the field of genetic screening
Classification kind code
11
Mark Details
Serial Number
1895446
Mark Type
Trademark
Legal History
| Action Taken | Status |
|---|---|
| Submitted for opposition 190 on 11th Nov 2020 | Registration Pending |
| Submitted for opposition 42 on 19th Aug 2020 | Advertised |
| Submitted for opposition 27 on 23rd Jul 2020 | Approval Notice Sent |
| Submitted for opposition 26 on 23rd Jul 2020 | Approved |
| Submitted for opposition 15 on 25th Feb 2020 | Correspondence Created |
| Submitted for opposition 22 on 15th Nov 2019 | Search Recorded |
| Submitted for opposition 20 on 15th Nov 2019 | Examiner's First Report |
| Submitted for opposition 31 on 27th Apr 2018 | Formalized |
| Submitted for opposition 1 on 25th Apr 2018 | Created |
| Submitted for opposition 30 on 24th Apr 2018 | Filed |