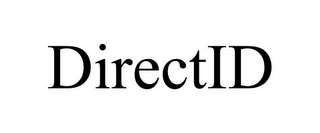| ITCH INSIGHT Advancing Innovation in Dermatology, Inc.
98001555 17 May 2023 | on 15 Oct 2024
| Providing a website featuring information on skin itch sensations Class 044 Class 044 Medical, Beauty & Agricultural Services The mark consists of the word "ITCH" in capital letters followed by a partial circle resembling a "C" shape with four smaller partial circles within (each in decreasing size) and next to which is another partial circle with two smaller partial circles within (also in decreasing size) also resembling a "C" shape but inverted and angled so that the smaller "C" shape opens facing the bottom of the larger "C" shape, all of which is followed by the word "INSIGHT" in capital letters and aligned with the word "ITCH" that is at the beginning of the mark. | | | DIRECTID MedPro Systems, LLC
86323921 30 Jun 2014 | on 13 Sep 2024
| Electronic matching services combining proprietary algorithms and othe... Class 035 Class 035 Advertising, Business & Retail Services DIRECT ID | | | HARVEY LIPSITZ CO. Champion Container Corporation
98039891 13 Jun 2023 | on 30 Jul 2024
| Distributorship services featuring bottles, jars, pails, cans, drums a... Class 035 Class 035 Advertising, Business & Retail Services "CO." | | | MEDPRO SYSTEMS MedPro Systems LLC
98048406 19 Jun 2023 | on 02 Jul 2024
| Software as a service (SaaS) services featuring software for use to as... Class 035 Class 035 Advertising, Business & Retail Services Business consulting services for life sciences, pharmaceuticals, biotechnology and medical device companies in the nature of regulatory compliance program development and enhancement and transparency program support, namely, reviewing program status and performing gap analyses, drafting programs plans, program implementation support, development and improvement of policies, standard operating procedures and codes of conduct; business consulting services in the nature of monitoring of pharmaceutical and medical device manufacturer interactions with healthcare professionals, healthcare organizations, patients, and other customers; business consulting services in the nature of auditing of pharmaceutical and medical device manufacturer interactions with and payments to healthcare professionals, healthcare organizations, patients, and other customers; business consulting services in the nature of business investigation support for internally and externally raised complaints made against a pharmaceutical or medical device manufacturer related to potential violations of their company policy or code, industry code, or law or regulation; transparency reporting regulatory submission management, namely, assisting others in preparing and filing regulatory submissions in the field of transparency reporting for pharmaceutical, biotechnology and medical device companies; business consulting services, namely, tracking and monitoring regulatory requirements for others in the field of life sciences, pharmaceuticals, biotechnology, and medical devices for regulatory compliance; business consulting services, namely, compliance program gap analysis for life sciences, pharmaceutical, biotechnology, and medical device companies Class 041 Class 041 Education and Entertainment Services Regulatory compliance consulting in the field of life sciences, pharmaceuticals, biotechnology, and medical devices; consulting services to assist life sciences, pharmaceutical, biotechnology and medical device companies in the fields of regulatory compliance, namely, compliance in the areas of aggregate spend disclosure reporting, the Prescription Drug Marketing Act, the Drug Supply Chain Security Act, Condition of Participation for Outpatient Services, Stark Law and Ohio Terminal Distributor of Dangerous Drugs requirements; regulatory compliance audits for life sciences, pharmaceutical, biotechnology and medical device companies to assist with regulatory compliance; consulting services in the field of life sciences, pharmaceutical, biotechnology and medical device corporate compliance program and transparency program development, implementation and support; providing online searchable databases and information concerning historical, current and pending laws, regulations, and guidance documents governing the life sciences industry; regulatory compliance consulting services in the field of aggregate spend disclosure reporting; regulatory and compliance information for pharmaceutical, biotechnology and medical device companies, and for entities involved in the drug and device supply chain, regarding transparency and aggregate spend, marketing, and additional compliance requirements; regulatory compliance consulting, namely, reviewing, developing, training on and assisting with the implementation and monitoring of standards, practices, procedures and policies to support regulatory compliance for companies in the fields of life sciences, pharmaceuticals, biotechnology, and medical devices Class 042 Computer & Software Services & Scientific Services "SYSTEMS"
Class 045 Personal & Legal & Social Services Software as a service (SaaS) services featuring software for use to assist in tracking and reporting healthcare practitioner and healthcare organization interactions and complying with international, federal, state and local spend and transparency laws and regulations; software as a service (SaaS) services featuring software to assist pharmaceutical, biotechnology and medical device companies in managing, validating and enriching healthcare organization and healthcare practitioner data attributes in connection with regulatory compliance, sales and marketing efforts | | | EXPERT ADVICE. SMART SOLUTIONS. COMPLIANCE SUCCESS. MedPro Systems LLC
98024973 02 Jun 2023 | on 25 Jun 2024
| Regulatory compliance consulting in the field of life sciences, pharma... Class 035 Class 035 Advertising, Business & Retail Services Consulting services in the nature of conducting trainings for life sciences, pharmaceuticals, biotechnology, and medical device companies personnel and partners on pharmaceutical and medical device compliance program requirements, fraud and corruption laws and regulations, industry codes and best practices; consulting services, namely, developing and providing trainings for life sciences, pharmaceutical, biotechnology, and medical device companies in the field of corporate compliance and transparency programs Class 041 Class 041 Education and Entertainment Services Business consulting services for life sciences, pharmaceuticals, biotechnology and medical device companies in the nature of regulatory compliance program development and enhancement and transparency program support, namely, reviewing program status and performing gap analyses, drafting programs plans, program implementation support, development and improvement of policies, standard operating procedures and codes of conduct; business consulting services in the nature of monitoring of pharmaceutical and medical device manufacturer interactions with healthcare professionals, healthcare organizations, patients, and other customers; business consulting services in the nature of auditing of pharmaceutical and medical device manufacturer interactions with and payments to healthcare professionals, healthcare organizations, patients, and other customers; business consulting services in the nature of business investigation support for internally and externally raised complaints made against a pharmaceutical or medical device manufacturer related to potential violations of their company policy or code, industry code, or law or regulation; transparency reporting regulatory submission management, namely, assisting others in preparing and filing regulatory submissions in the field of transparency reporting for pharmaceutical, biotechnology and medical device companies; business consulting services, namely, tracking and monitoring regulatory requirements for others in the field of life sciences, pharmaceuticals, biotechnology, and medical devices for regulatory compliance; business consulting services, namely, compliance program gap analysis for life sciences, pharmaceutical, biotechnology, and medical device companies Class 045 Personal & Legal & Social Services Regulatory compliance consulting in the field of life sciences, pharmaceuticals, biotechnology, and medical devices; consulting services to assist life sciences, pharmaceutical, biotechnology and medical device companies in the fields of regulatory compliance, namely, compliance in the areas of aggregate spend disclosure reporting, the Prescription Drug Marketing Act, the Drug Supply Chain Security Act, Condition of Participation for Outpatient Services, Stark Law and Ohio Terminal Distributor of Dangerous Drugs requirements; compliance audits in the field of life sciences, pharmaceutical, biotechnology and medical device companies regulatory compliance; consulting services in the field of life sciences, pharmaceutical, biotechnology and medical device corporate compliance program and transparency program development, implementation and support; regulatory compliance consulting, namely, reviewing, developing, training on and assisting with the implementation and monitoring of standards, practices, procedures and policies to support regulatory compliance for companies in the fields of life sciences, pharmaceuticals, biotechnology, and medical device | | | PHLIP LUNCH Jane and Frances Stein Foundation
90078078 29 Jul 2020 | on 02 Apr 2024
| Promoting public awareness of childhood hunger and school lunch availa... Class 035 Class 035 Advertising, Business & Retail Services Promoting public awareness of childhood hunger and school lunch availably and affordability issues Class 036 Class 036 Insurance & Financial Services "LUNCH" | | | HEARTS AND HANDS Hearts and Hands Professional Development & Educational Consulting LLC
97677554 15 Nov 2022 | on 06 Feb 2024
| Educational consulting services, namely, conducting programs, seminars... Class 041 Class 041 Education and Entertainment Services Educational consulting services, namely, conducting programs, seminars, workshops classes and trainings and in-person forums for school educators, school administrators, school counselors, and school paraprofessionals in the fields of professional development, social and emotional learning and literacy and the distribution of educational material in connection therewith; Educational consulting services, namely, conducting workshops for parents and other caregivers of children in the fields of social and emotional learning and literacy and the distribution of educational material in connection therewith | | | CHALLENGEID MedPro Systems LLC
87075647 17 Jun 2016 | on 20 Dec 2023
| Electronic matching services in the nature of matching eligible health... Class 035 Class 035 Advertising, Business & Retail Services Electronic matching services in the nature of matching eligible healthcare providers to pharmaceutical companies and distributors for business purposes by providing on-demand verification of the healthcare practitioner and healthcare practitioner's licensing status through use of proprietary algorithms, and other mathematical operations with a database containing healthcare practitioners' information based on questions derived from the practitioner's current and historical license history | | | Logo Mark MedPro Systems LLC
97448093 08 Jun 2022 | on 12 Sep 2023
| Regulatory compliance consulting in the field of life sciences, pharma... Class 035 Class 035 Advertising, Business & Retail Services Color is not claimed as a feature of the mark. Class 041 Class 041 Education and Entertainment Services Business consulting services for life sciences, pharmaceuticals, biotechnology and medical device companies in the nature of regulatory compliance program development and enhancement and transparency program support, namely, reviewing program status and performing gap analyses, drafting programs plans, program implementation support, development and improvement of policies, standard operating procedures and codes of conduct; business consulting services in the nature of monitoring of pharmaceutical and medical device manufacturer interactions with healthcare professionals, healthcare organizations, patients, and other customers; business consulting services in the nature of auditing of pharmaceutical and medical device manufacturer interactions with and payments to healthcare professionals, healthcare organizations, patients, and other customers; business consulting services in the nature of business investigation support for internally and externally raised complaints made against a pharmaceutical or medical device manufacturer related to potential violations of their company policy or code, industry code, or law or regulation; transparency reporting regulatory submission management, namely, assisting others in preparing and filing regulatory submissions in the field of transparency reporting for pharmaceutical, biotechnology and medical device companies; business consulting services, namely, tracking and monitoring regulatory requirements for others in the field of life sciences, pharmaceuticals, biotechnology, and medical devices for regulatory compliance; business consulting services, namely, compliance program gap analysis for life sciences, pharmaceutical, biotechnology, and medical device companies Class 045 Personal & Legal & Social Services Regulatory compliance consulting in the field of life sciences, pharmaceuticals, biotechnology, and medical devices; consulting services to assist life sciences, pharmaceutical, biotechnology and medical device companies in the fields of regulatory compliance, namely, compliance in the areas of aggregate spend disclosure reporting, the Prescription Drug Marketing Act, the Drug Supply Chain Security Act, Condition of Participation for Outpatient Services, Stark Law and Ohio Terminal Distributor of Dangerous Drugs requirements; compliance audits in the field of life sciences, pharmaceutical, biotechnology and medical device companies regulatory compliance; consulting services in the field of life sciences, pharmaceutical, biotechnology and medical device corporate compliance program and transparency program development, implementation and support; regulatory compliance consulting, namely, reviewing, developing, training on and assisting with the implementation and monitoring of standards, practices, procedures and policies to support regulatory compliance for companies in the fields of life sciences, pharmaceuticals, biotechnology, and medical devices | | | MEDPRO COMPLIANCE ADVISORY SERVICES MedPro Systems LLC
97310307 14 Mar 2022 | on 12 Sep 2023
| Regulatory compliance consulting in the field of life sciences, pharma... Class 035 Class 035 Advertising, Business & Retail Services Business consulting services for life sciences, pharmaceuticals, biotechnology and medical device companies in the nature of regulatory compliance program development and enhancement and transparency program support, namely, reviewing program status and performing gap analyses, drafting programs plans, program implementation support, development and improvement of policies, standard operating procedures and codes of conduct; business consulting services in the nature of monitoring of pharmaceutical and medical device manufacturer interactions with healthcare professionals, healthcare organizations, patients, and other customers; business consulting services in the nature of auditing of pharmaceutical and medical device manufacturer interactions with and payments to healthcare professionals, healthcare organizations, patients, and other customers; business consulting services in the nature of business investigation support for internally and externally raised complaints made against a pharmaceutical or medical device manufacturer related to potential violations of their company policy or code, industry code, or law or regulation; transparency reporting regulatory submission management, namely, assisting others in preparing and filing regulatory submissions in the field of transparency reporting for pharmaceutical, biotechnology and medical device companies; business consulting services, namely, tracking and monitoring regulatory requirements for others in the field of life sciences, pharmaceuticals, biotechnology, and medical devices for regulatory compliance; business consulting services, namely, compliance program gap analysis for life sciences, pharmaceutical, biotechnology, and medical device companies Class 041 Class 041 Education and Entertainment Services Regulatory compliance consulting in the field of life sciences, pharmaceuticals, biotechnology, and medical devices; consulting services to assist life sciences, pharmaceutical, biotechnology and medical device companies in the fields of regulatory compliance, namely, compliance in the areas of aggregate spend disclosure reporting, the Prescription Drug Marketing Act, the Drug Supply Chain Security Act, Condition of Participation for Outpatient Services, Stark Law and Ohio Terminal Distributor of Dangerous Drugs requirements; compliance audits in the field of life sciences, pharmaceutical, biotechnology and medical device companies regulatory compliance; consulting services in the field of life sciences, pharmaceutical, biotechnology and medical device corporate compliance program and transparency program development, implementation and support; regulatory compliance consulting, namely, reviewing, developing, training on and assisting with the implementation and monitoring of standards, practices, procedures and policies to support regulatory compliance for companies in the fields of life sciences, pharmaceuticals, biotechnology, and medical devices Class 045 Personal & Legal & Social Services "COMPLIANCE ADVISORY SERVICES" | |