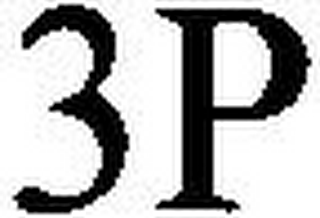
PARTIAL SECTION 71 ACCEPTED
on 19 Sep 2022
Last Applicant/ Owned by
MARCY L'ETOILE
FR
F-69280
Serial Number
79144266 filed on 06th Nov 2013
Registration Number
4690345 registered on 24th Feb 2015
Filing Basis
1. filing basis filed as 66 a
Disclaimer
NO DATA
3P
[ Internal quality control for others in laboratories and industry; Inter-laboratory and inter-industry quality control for others; Assistance in approaching quality in accordance with current regulations, standards and guidelines, namely, technology supervision and inspection in the field of product quality control; Consulting in the field of chemical, biochemical, biological, and bacteriological Read More
Classification Information
Class [042]
Computer & Software Services & Scientific Services
[ Internal quality control for others in laboratories and industry; Inter-laboratory and inter-industry quality control for others; Assistance in approaching quality in accordance with current regulations, standards and guidelines, namely, technology supervision and inspection in the field of product quality control; Consulting in the field of chemical, biochemical, biological, and bacteriological research and analysis, namely, as it relates to the technical and scientific implementation and improvement of the aforesaid quality control services; Quality control services for others, namely, environmental monitoring and control particularly of physical and environmental parameters inside laboratories or industry or outside of laboratories or industry, namely, detecting and recording parameters such as temperature, pressure, humidity, presence of gas, operating time and other parameters in laboratories or industry, and outside of laboratories or industry for quality control purposes ]
Class [009]
Computer & Software Products & Electrical & Scientific Products
[ Laboratory glassware, namely, petri dishes, tubes, and vials for culture media intended for in-vitro diagnosis; ] Scientific apparatus and instruments for collecting, monitoring and detecting contaminants in industrial, pharmaceutical, cosmetic and agri-food products and in the production environment of these products, namely, in water, air, and on working surfaces [ ; Software for non-medical diagnosis purposes, namely, for collecting, monitoring and detecting contaminants in industrial, pharmaceutical, cosmetic and agri-food products and in the production environment of these products, namely, in water, air, and on working surfaces, with the aforementioned software being for laboratory and research use; Scientific apparatus and instruments for in-vitro diagnosis not for medical use, namely, for monitoring and tracking of samples, reagents and collected data; Software for monitoring and tracking of laboratory samples, reagents and collected data for scientific research purposes; Measuring and monitoring apparatus and instruments enabling the recording of physical and environmental parameters inside medical laboratories or outside of medical laboratories as well as inside and outside of industry, namely, immune analyzers, nucleic acid analyzers, bacteriological analyzers, microbiologic analyzers; Software for processing and following-up data for monitored parameters, namely, for recording and tracking environmental parameter data from medical laboratories or outside of medical laboratories as well as inside and outside of industrial locations; Application software for the aforementioned scientific instruments in the field of diagnostics, namely, software for collecting, recording, and processing environmental data ]
Class [005]
Pharmaceutical Products
[ Diagnostic reagents for medicinal use, namely, for medical, pharmaceutical, and veterinary in-vitro diagnosis; Cell growth media for growing cells for clinical medical, clinical pharmaceutical, and clinical veterinary purposes, namely, for in-vitro diagnosis purposes ]
Class [001]
Chemical Products
Nutrient media for [in-vitro diagnosis, ] monitoring and detecting contaminants in industrial, pharmaceutical, agri-food products and in the production environment of these products, namely, in water, air, and on working surfaces [ ; Chemical and biochemical products enabling quality control of reagents and systems used in laboratories and industry, namely, colorimetric chemical compounds for analyzing the presence of biological substances and test particles for bio-detection systems ]
Mark Details
Serial Number
No 79144266
Mark Type
No Service Mark
Attorney Docket Number
No
44D Filed
No
44D Current
No
44E filed
No
44E Current
No
66A Filed
Yes
66A Current
Yes
Current Basis
No
No Basis
No
Legal History
| Status Date | Action Taken |
|---|---|
| 24th Feb 2024 | COURTESY REMINDER - SEC. 71 (10-YR) E-MAILED |
| 25th Jul 2023 | PARTIAL INVALIDATION PROCESSED BY THE IB |
| 23rd Jun 2023 | INTERNATIONAL REGISTRATION RENEWED |
| 20th Jun 2023 | INVALIDATION PROCESSED |
| 20th Jun 2023 | PARTIAL INVALIDATION OF REG EXT PROTECTION SENT TO IB |
| 19th May 2023 | PARTIAL INVALIDATION OF REG EXT PROTECTION CREATED |
| 19th Sep 2022 | POST REGISTRATION ACTION MAILED NO RESPONSE REQUIRED |
| 19th Sep 2022 | REGISTERED - PARTIAL SEC 71 ACCEPTED |
| 19th Sep 2022 | NOTICE OF ACCEPTANCE OF SEC. 71 - E-MAILED |
| 16th Sep 2022 | POST REGISTRATION ACTION MAILED - SEC.71 |